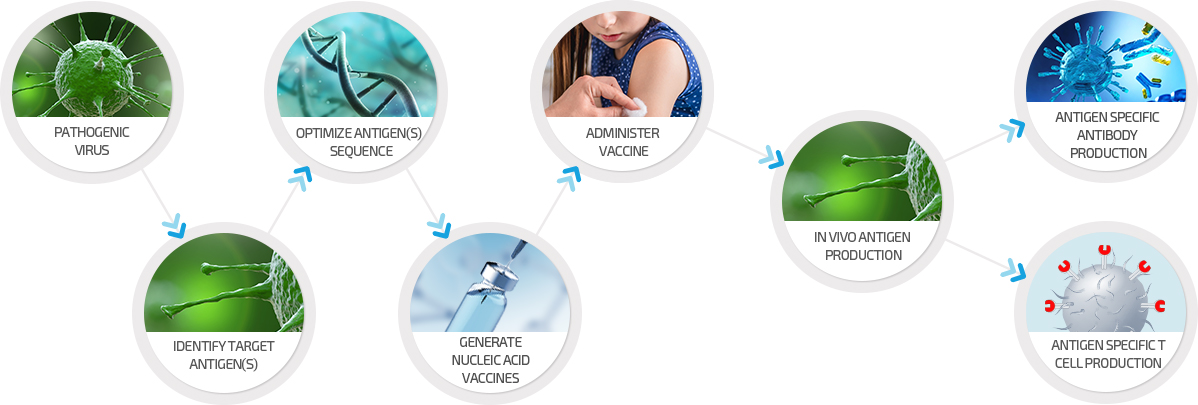
Nucleic Acid Vaccines
Vaccines are a powerful tool to prevent infectious diseases.
GeneOne Life Science can rapidly develop its DNA vaccines.
GeneOne’s patented manufacturing technology, which has been developed over several years at VGXI, enables the rapid development and manufacture of large quantities of cGMP grade vaccine.
With the increasing occurrence of emerging new viral infectious diseases, GeneOne can leverage its nucleic acid-based vaccine platform to rapidly address new lethal diseases.
- Principle
-

GLS-3000 Prevention of COVID-19 disease
-
gls-5100 Prevention of herpes zoster (shingles)
-

gls-5140 Prevention of severe fever with thrombocytopenia syndrome (SFTS)
-

gls-5300 Prevention of Middle East respiratory syndrome coronavirus (MERS-CoV) infection
-
GLS-5310 DNA Prevention of COVID-19 disease
-

gls-5700 Prevention of Zika virus infection
-

gls-6100
gls-6150 Prevention of hepatitis C
GLS-5100 Prevention of herpes zoster (shingles)
- Product Information
- GLS-5100 is a DNA vaccine against varicella zoster virus (VZV) encoding an antigen involved in virus latency. GLS-5100 induces no safety concerns and can be used in immune suppressed persons, unlike other commercial vaccines.
- Disease Condition
- Shingles is caused by Varicella Zoster virus (VZV), a member of the Herpes virus family that also causes chicken pox. Shingles is the result of reactivation of VZV along the sensory nerve causing a painful blistering rash. The risk of shingles increases as you get older or develop other disorders, such as cancer, that weaken the immune system. About 1 out of every 3 people will develop shingles in their lifetime and 10-18% of those will develop post-herpetic neuralgia, persistent pain after infection clears. (1)
- Development State
-
GeneOne is developing the GLS-5100 VZV-Shingles DNA vaccine as an alternative and more affordable option to the live-virus and recombinant VZV vaccines currently on the market in the US. (2) GLS-5100 is in pre-clinical development.
- https://www.cdc.gov/shingles/index.html
- https://www.cdc.gov/shingles/vaccination.html
- Performance
-
- Immune Netw. (2018) 18(5): e38
GLS-5140 Prevention of severe fever with thrombocytopenia syndrome (SFTS)
- Product Information
- GLS-5140 is a DNA vaccine designed to prevent infections by Dabie bandavirus (formerly SFTS virus (SFTSV)).
- Disease Condition
- The Severe Fever and Thrombocytopenia Syndrome virus (SFTSV) is a tick-borne virus that causes a hemorrhagic illness that is between 20% and 40% fatal (1). Initially recognized in 2009 in China (2), cases of SFTSV have occurred at increasing numbers over the past decade in Korea, China, and Japan. In Korea, cases of SFTSV have been diagnosed in all provinces with the highest number in the southern provinces, especially Jeju island. (3)
- Development State
-
GeneOne is developing the GLS-5140 SFTSV DNA vaccine as part of our preparedness efforts in Emerging Infectious Disease. With the incidence of SFTSV on the rise, we are focused on making a safe and effective vaccine that can be available should the spread of SFTSV expand. Initial GLS-5140 pre-clinical experiments have shown the vaccine candidate induced both a neutralizing antibody response and multifunctional SFTSV-specific T-cell response in mice and ferrets. (4) Importantly, ferrets vaccinated with GLS-5140 remained healthy after SFTSV challenge that was 100% fatal in control animals. (4) GLS-5140 is in pre-clinical development.
- – Maslow 2019,
- Yu 2011 NEJM,
- Yoo 2016,
- Kwak 2019
- Performance
-
- Nat Commun. (2019) 10:3836
GLS-5300
Prevention of Middle East respiratory syndrome coronavirus (MERS-CoV) infection
- Product Information
- GLS-5300 is a DNA vaccine encoding for the whole spike protein of the MERS-coronavirus (MERS-CoV)
- Disease Condition
- Middle East respiratory syndrome (MERS) coronavirus is a highly fatal cause of lower respiratory tract infection first identified in 2012. (1) As of January 2020, over 2500 laboratory-confirmed cases of MERS have been reported across 27 countries of whom 34% died. In 2015, an infected traveler returning to South Korea from the Middle East resulted in 186 cases of MERS of whom 20% died. There are no licensed vaccines to prevent or therapeutics to treat MERS coronavirus infection.
- Development State
-
There are no licensed vaccines to prevent or therapeutics to treat MERS-CoV infection.
In 2019, GeneOne published in The Lancet Infectious Disease results of its phase I trial (NCT02670187) of the GLS-5300 MERS-CoV DNA vaccine. The study showed that the vaccine was well tolerated and generated both antibody-based and cellular immune responses that were similar to immune responses of Korean patients who had recovered from natural MERS infection during the 2015 outbreak.
GeneOne and the International Vaccine Institute have recently completed a Phase 1/2a study of GLS-5300 (NCT03721718) in Korea.- Corman 2012, 2. www.emro.who.int/health-topics/mers-cov/mers-outbreaks.html, 3. Modjarrad 2019
- 성과
-
- Lancet Infect Dis. (2019) 10.1016/S1473-3099(19)30397-4
GLS-5700 Prevention of Zika virus infection
- Product Information
- GLS-5700 is a DNA vaccine encoding the pre-membrane and envelop (prM-E) proteins of Zika virus (ZIKV).
- Disease Condition
- Zika virus (ZIKV) is a flavivirus that was originally discovered in Uganda in 1947 (1). ZIKV infection was identified in Brazil in 2015 and spread rapidly throughout the Americas. (2) ZIKV infection is typically mild but is associated with neurologic syndromes such as Guillain-Barré and microcephaly in infants of women infected while pregnant. (3, 4) ZIKV is spread by the bite of infected mosquitoes, but has also been transmitted through sexual contact, blood transfusion, and laboratory exposures. There are no approved ZIKV-specific therapies or vaccines.
- Development State
-
In 2017, we published in the New England Journal of Medicine the results of our phase 1 clinical trial (NCT02809443) of the GLS-5700 ZIKV DNA vaccine. We showed that 100% of participants developed antibodies. Importantly, serum from vaccinated study participants protected immune-suppressed mice against lethal ZIKV infection. A second phase 1 study of GLS-5700 was conducted in Puerto Rico (NCT02887482), data analysis is ongoing.
- Dick 1952, 2. Campos 2015, 3. Brasil 2016, 4. Song 2017,
- Performance
-
- N Engl J Med. (2017) doi: 10.1056/NEJMoa1708120.
- NPJ Vaccines. 2016 Nov 10;1:16021. doi: 10.1038/npjvaccines.2016.21.
GLS-6100/GLS-6150 Prevention of hepatitis C
- Product Information
- GeneOne has two DNA vaccines targeting hepatitis C virus (HCV) infections: one to prevent infection in people never exposed to HCV (GLS-6100) and one to prevent re-infection in those who were previously HCV infected and were cured (GLS-6150). Both GLS-6100 and GLS-6150 target multiple HCV proteins to prevent its growth GLS-6150 has an additional immuno-adjuvant designed to reverse immune tolerance occurring with HCV infection that is not reversed after cure. With our duo of HCV DNA vaccines, we seek to provide an efficacious, safe and cost-effective means to prevent and treat HCV in all at-risk individuals.
- Disease Condition
-
Hepatitis C virus (HCV) causes liver infection that may lead to hepatic cirrhosis, liver failure or liver cancer (hepatocellular carcinoma). Hepatitis C is mostly spread through contaminated blood, although sexual transmission may also occur. Approximately 1-2% of the populations of the US and Korea are infected with HCV. (1) For 70–85% of people who become infected, HCV becomes a long-term, chronic infection and 15-30% may develop more severe complications including liver cancer. (2)
There is no vaccine for HCV, but new direct acting antiviral drug therapies can treat the infection and provide a cure for most, but these treatments can cost $40,000 - $100,000 USD per course. (3) People who have had HCV and either cleared the virus or have been treated and cured can become re-infected with HCV.- https://www.cdc.gov/hepatitis/hcv/index.htm
- https://www.cdc.gov/hepatitis/hcv/cfaq.htm#statistics
- https://www.hepatitisc.uw.edu/page/treatment/drugs
- Development State
- GLS-6100 will be evaluated in an upcoming phase I clinical trial of HCV-naïve healthy adults in Korea. GLS-6150 was tested in a phase I trial (NCT02027116) of HCV-infected individuals who failed prior interferon-based therapy. GLS-6150 is currently being evaluated in a second phase I clinical trial in Korea (NCT03674125) in previously HCV-infected individuals who were successfully treated and cured.
- Performance
-
- Sci. Rep. (2017) e7
GLS-3000(mRNA) Prevention of COVID-19 disease
- Product Information
- GLS-3000 is an RNA vaccine encoding for the spike protein (S) of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
- Disease Condition
- SARS-CoV-2, the virus that causes COVID-19 disease, has led to unprecedented disruption to the social and economic lives of people worldwide. The death rate has been estimated to be anywhere from 1-7%, with people of advanced age and with preexisting conditions most susceptible to poor outcomes.
- Development State
- GLS-3000 is being developed as a collaboration between GeneOne and Houston Methodist Research Institute (Houston, USA), a world leading research institute in mRNA medicine. GLS-3000 is in pre-clinical development.
- Performance
GLS-5310(DNA) Prevention of COVID-19 disease
- Product Information
- GLS-5310 is a DNA vaccine encoding the spike (S) protein and a second antigenic target of SARS-CoV-2.
- Disease Condition
- SARS-CoV-2, the virus that causes COVID-19 disease, has led to unprecedented disruption to the social and economic lives of people worldwide. The death rate has been estimated to be anywhere from 1-7%, with people of advanced age and with preexisting conditions most susceptible to poor outcomes.
- Development State
- GLS-5310 is being developed with support from the Government of Korea, through the Centers for Disease Control and Prevention, as part of a national project to develop COVID-19 vaccines. GLS-5310 is in pre-clinical development.
- Performance
GLS-5310(DNA) Prevention of coronavirus disease 2019 (COVID-19)
- Product Information
- GLS-5310 is a DNA vaccine encoding the spike (S) protein and a second antigenic target of SARS-CoV-2.
- Disease Condition
- SARS-CoV-2, the virus that causes COVID-19 disease, has led to unprecedented disruption to the social and economic lives of people worldwide. The death rate has been estimated to be anywhere from 1-7%, with people of advanced age and with preexisting conditions most susceptible to poor outcomes.
- Development State
- GLS-5310 is being developed with support from the Government of Korea, through the Centers for Disease Control and Prevention, as part of a national project to develop COVID-19 vaccines. GLS-5310 is in pre-clinical development.
- Performance
GLS-7000 HER2-targeted mAb Drug Resistant Breast Cancer
- Product Information
- GLS-7000 is a DNA-based recombinant protein therapeutic (dRP). It is a bi-specific protein designed to target and bind two factors involved in many breast cancers. GLS-7000, as a treatment against both HER2-susceptible and HER2-resistant breast cancer, is in the discovery and optimization stage.
- Disease Condition
-
Each year in the United States, approximately 245,000 cases of breast cancer are diagnosed in women and 2,200 in men. About 41,000 women and 460 men in the U.S. die each year from breast cancer. African-American women have a higher rate of death from breast cancer than Caucasian women and the rate has increased for Asian and Pacific Islander women. (1) Approximately 25% of breast cancers overexpress the human epidermal growth factor receptor 2 (HER2) protein on the surface of breast cancer cells. Anti-HER2 monoclonal antibodies are a mainstay of treatment against such tumors, but many women develop resistance over time. (2)
- https://www.cdc.gov/cancer/breast/basic_info/index.htm
- https://www.cdc.gov/cancer/breast/basic_info/treatment.htm
- Development State
- GLS-7000, as a treatment against both HER2-susceptible and HER2-resistant breast cancer, is in the discovery and optimization stage.
- Performance
-
- Exp Mol Med. (2013) 45, e52; doi:10.1038/emm.2013.111
GLS-7100 Hemophilia A
- Product Information
- GLS-7100 is a DNA-based recombinant factor VIII treatment designed to allow Hemophilia A sufferers to make factor VIII in their own cells and is expected to be an effective and cost-efficient alternative to current therapies.
- Disease Condition
-
Hemophilia A is an X-linked bleeding disorder resulting from mutations in the gene encoding for coagulation factor VIII, a critical protein in the blood clotting cascade. Patients with severe hemophilia A are susceptible to spontaneous bleeding, reduced quality of life, and increased risks of bleeding in the brain and early death. (1)
Patients with hemophilia A are often treated by replacement through injections or infusions of either serum-derived or recombinant factor VIII. Current treatments are expensive and, for serum-derived replacements, can carry the risk for blood-borne illness. Other available treatments include monoclonal antibody therapy and medications that release natural factor VIII or prevent clot degradation. (2) Although a gene therapy for Hemophilia A has been approved, the fraction of patients who can receive this is limited.- http://helpline.nih.go.kr/cdchelp/ph/rdiz/selectRdizInfDetail.do?menu=A0100&rdizCd=RA201710128
- 2018 White Paper on Hemophilia, Korea Hemophilia Foundation
- Development State
- GLS-7100 is in the discovery and optimization stage.
GLS-8000 Prevention of Chronic Hepatitis B/Hepatitis B
- Product Information
- GLS-8000 is a DNA-based plasmid monoclonal antibody (Plas-mAb) therapeutic that encodes monoclonal antibodies designed to target critical viral antigens to block progression or infection of hepatitis B virus (HBV).
- Disease Condition
-
Hepatitis B virus (HBV) is a viral infection that causes both acute and chronic liver disease. Despite the availability of vaccination in over 100 countries, HBV infection remains a severe problem worldwide. In 2016, the WHO estimated that 257 million people suffered from chronic hepatitis B and 887,000 people succumbed to death after HBV-related cirrhosis and liver cancer. (1,2)
Effective prevention of mother-to-child transmission of HBV requires both HBV vaccination and the administration of hepatitis-B immune globulin (HBIG) at birth. HBIG is expensive to make and may carry the risk for transmission of other bloodborne diseases. While recombinant monoclonal antibodies have replaced HBIG in developed countries, they are expensive and largely unavailable to the developing world.
HBIG or HBV monoclonal antibodies are also critical to prevent HBV infection following needle-stick injuries in healthcare workers and for those receiving liver transplants from those who previously had HBV infection- World Health Organization, Global Hepatitis B Report 2017. https://www.who.int/en/news-room/fact-sheets/detail/hepatitis-b (2017)
- https://www.cdc.gov/hepatitis/hbv/index.htm
- Development State
- GLS-8000 is in the discovery and optimization stage.
- Performance
GLS-8100 Rheumatoid Arthritis
- Product Information
- GLS-8100 is a DNA-based Plas-mAb therapeutic that codes for the production of a human IgG1 monoclonal antibody that specificity targets human tumor necrosis factor alpha (TNF-α).
- Disease Condition
-
Rheumatoid arthritis (RA) is an autoimmune and inflammatory disease that causes painful swelling mainly in the joints and usually in many joints at once such as hands, wrists, and knees. In a joint with RA, the lining becomes inflamed, causing damage to tissue and long-lasting or chronic pain, loss of balance, and deformity. Other tissues such as the heart and lungs can also be affected by inflammation caused by RA. (1) Treatments for RA include anti-inflammatory medications to slow progression, prevent pain, and joint deformity or biologicals as a second-line treatment. (2,3) Biologicals, such as monoclonal antibodies, are expensive and can be difficult to produce.
Tumor Necrosis Factor-α (TNF-α) is a component of the immune system that can drive inflammation when in excess. Elevated levels of TNF-α found in the joints of patients with RA contribute to the inflammation and joint destruction that are hallmarks of the disease. Monoclonal antibodies against TNF-α that reduce inflammation and prevent progression of RA are available. (2)- https://www.cdc.gov/arthritis/basics/rheumatoid-arthritis.html
- http://health.cdc.go.kr/health/HealthInfoArea/HealthInfo/View.do?idx=2190
- https://www.rheumatology.org/Practice-Quality/Clinical-Support/Clinical-Practice-Guidelines/Rheumatoid-Arthritis
- https://www.rheumatology.org/I-Am-A/Patient-Caregiver/Diseases-Conditions/Rheumatoid-Arthritis
- Development State
- GLS-8100 is in the discovery and optimization stage.
GLS-1027 Acute ischemic nephritis
- Product Information
- GLS-1027 is a synthetic small molecule drug with novel anti-inflammatory properties. It has exhibited an ability to reduce the production of multiple pro-inflammatory cytokine in a variety of preclinical animal models of inflammatory disease which has correlated with reduced morbidity in these studies.(1,2,3)
- Disease Condition
-
In 2017, it was estimated that over 451 million people worldwide have Diabetes Mellitus with the number expected to rise to 693 million by 2045 (1). The development of kidney disease is a serious complication in up to 30% of diabetics.
As diabetes progresses, inflammation leads to damage of cells in the kidneys called podocytes leading to a condition known as diabetic nephropathy (DN) and, ultimately, to end stage kidney failure. Inflammation also occurs when the blood flow to the kidneys is interrupted during heart surgery leading to acute kidney injury (AKI).- Cho 2018
- Saurus 2015
- Saurus 2016
- Development State
- GLS-1027 was safe and well tolerated in prior phase 1 clinical studies in man. (NCT00627120, NCT00760396) A phase 2 study of GLS-1027 for the prevention of nephropathy is planned.
- Performance
-
- Clin. Pharmacol. Drug Dev. (2016) e5(2)
GLS-1027 COVID-19 Pneumonitis
- Product Information
- GLS-1027 treatment significantly reduced levels of proinflammatory cytokines resulting in pulmonary disease in animal models (1). GLS-1027 was also able to lower or prevent lung disease caused by fine particulate matter in animal models (2).
- Disease Condition
-
SARS-CoV-2, the virus that causes COVID-19 disease, has led to unprecedented disruption to the social and economic lives of people worldwide. The death rate has been estimated to be anywhere from 1-7%, with people of advanced age and with preexisting conditions most susceptible to poor outcomes.
While SARS-CoV-2 infection manifests as an upper respiratory tract disease in most people, up to 20% of infected patients can progress to severe disease that starts as pneumonia, characterized in part by an infiltration of neutrophils and macrophages into the alveoli. This immune cell infiltration correlates with the appearance of a “cytokine storm” characterized by high levels of pro-inflammatory cytokines including IL-6, TNF-alpha and others in the lungs. Some patients who develop pneumonia progress to severe acute respiratory distress syndrome which has a poor prognosis and requires intubation and mechanical ventilation or ECMO to treat.- Zhang et al. (2015). Eur J Pharm. 765; 94-99.
- Xu et al. (2019). Eur J Pharm. 842; 373-383.
- Development State
- A phase 2 study of GLS-1027 for the prevention of severe pneumonitis in SARS-CoV-2 infection is planned
- Performance
GLS-1200 Chronic Rhinosinusitis
- Product Information
- GLS-1200 nasal spray stimulates multiple bitter taste receptors (TAS2Rs) on ciliated nasal epithelial cells to produce nitric oxide as part of the innate immune system. Nitric oxide has generalized anti-microbial activity in the upper respiratory tract.
- Disease Condition
- Chronic rhinosinusitis (CRS) affects around 15% of the population, is a significant burden on the quality of life for sufferers and costs the healthcare system over 8 billion USD annually.
- Development State
-
GLS-1200 is being developed with our academic partners at the University of Pennsylvania.
A phase 2 study of GLS-1200 nasal spray for prevention of recurrent infections in CRS patients following sinus surgery is in progress. (NCT04060316)
- Performance
GLS-1200 COVID-19 Prophylaxis
- Product Information
- GLS-1200 nasal spray stimulates multiple bitter taste receptors (TAS2Rs) on ciliated nasal epithelial cells to produce nitric oxide as part of the innate immune system. Nitric oxide has generalized anti-microbial activity in the upper respiratory tract.
- Disease Condition
- The nose is the primary portal of entry for coronaviruses that cause diseases that range in severity from the common cold to life threatening COVID-19 and Influenza. For COVID-19, the levels of virus in the nose can reach very high levels. In fact, transmission of COVID-19 can occur in asymptomatic individuals to others in their vicinity.
- Development State
-
GLS-1200 is being developed with our academic partners at the University of Pennsylvania.
GLS-1200 nasal spray is currently being tested as part of a Phase 2 study for prevention of COVID-19 infection in healthcare workers. (NCT04481183)
- Performance
GLS-1200 Influenza and Common cold viruses
- Product Information
- GLS-1200 nasal spray stimulates multiple bitter taste receptors (TAS2Rs) on ciliated nasal epithelial cells to produce nitric oxide as part of the innate immune system. Nitric oxide has generalized anti-microbial activity in the upper respiratory tract.
- Disease Condition
-
During the 2019-2020 flu season in the United states it is estimated that 39 – 56 million people were infected with the flu, and that 24,000 – 26,000 people died (1). In addition to the annual burden of the flu, adults in the US have on average 2-3 colds annually (2). The nose is the primary portal of entry for each of the common cold viruses including Influenza.
- https://www.cdc.gov/flu/about/burden/preliminary-in-season-estimates.htm
- https://www.cdc.gov/antibiotic-use/community/for-patients/common-illnesses/colds.html
- Development State
-
GLS-1200 is being developed with our academic partners at the University of Pennsylvania.
A phase 2 study of GLS-1200 nasal spray for the prevention of influenza and common cold viruses is planned.
- Performance